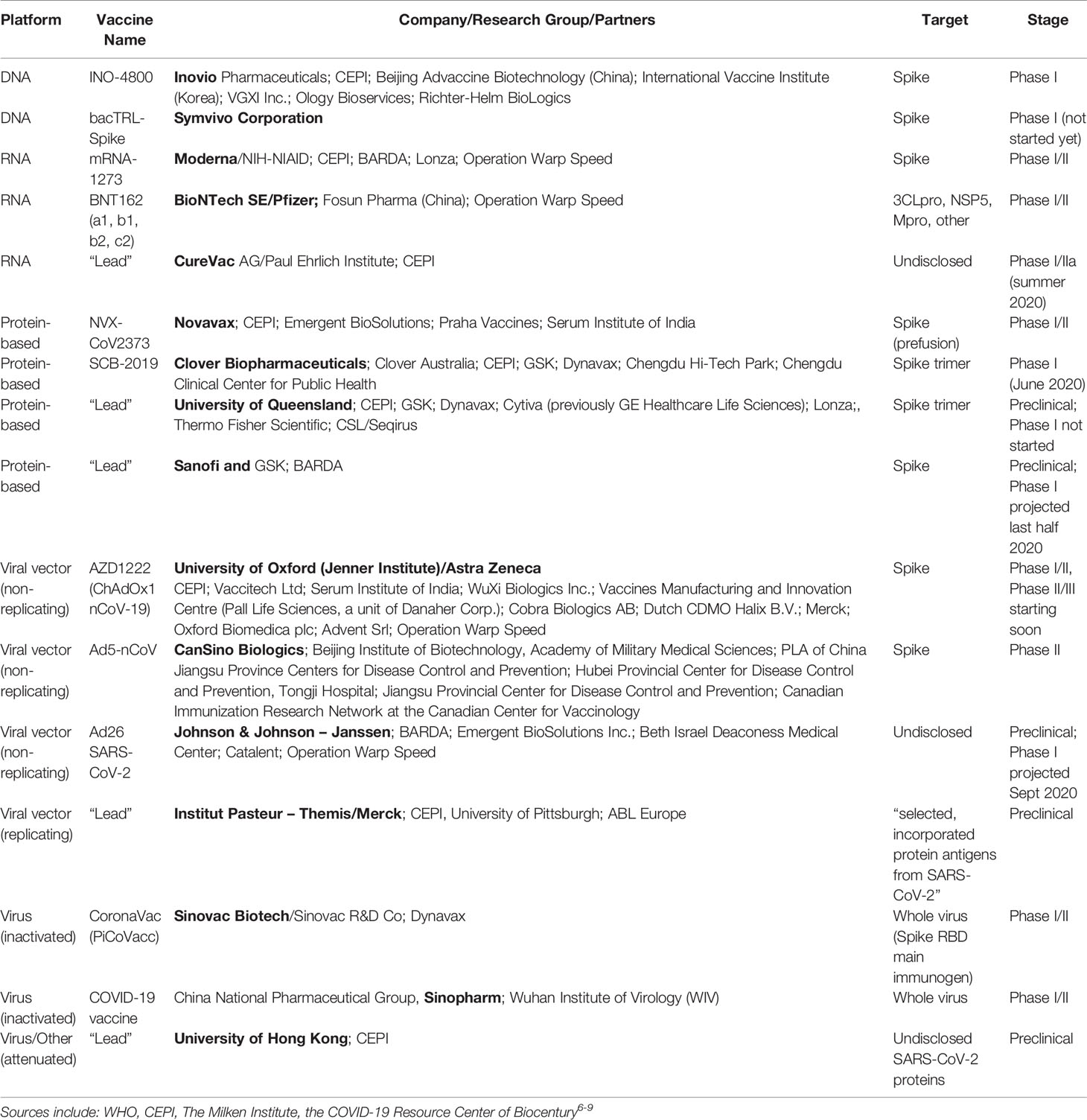
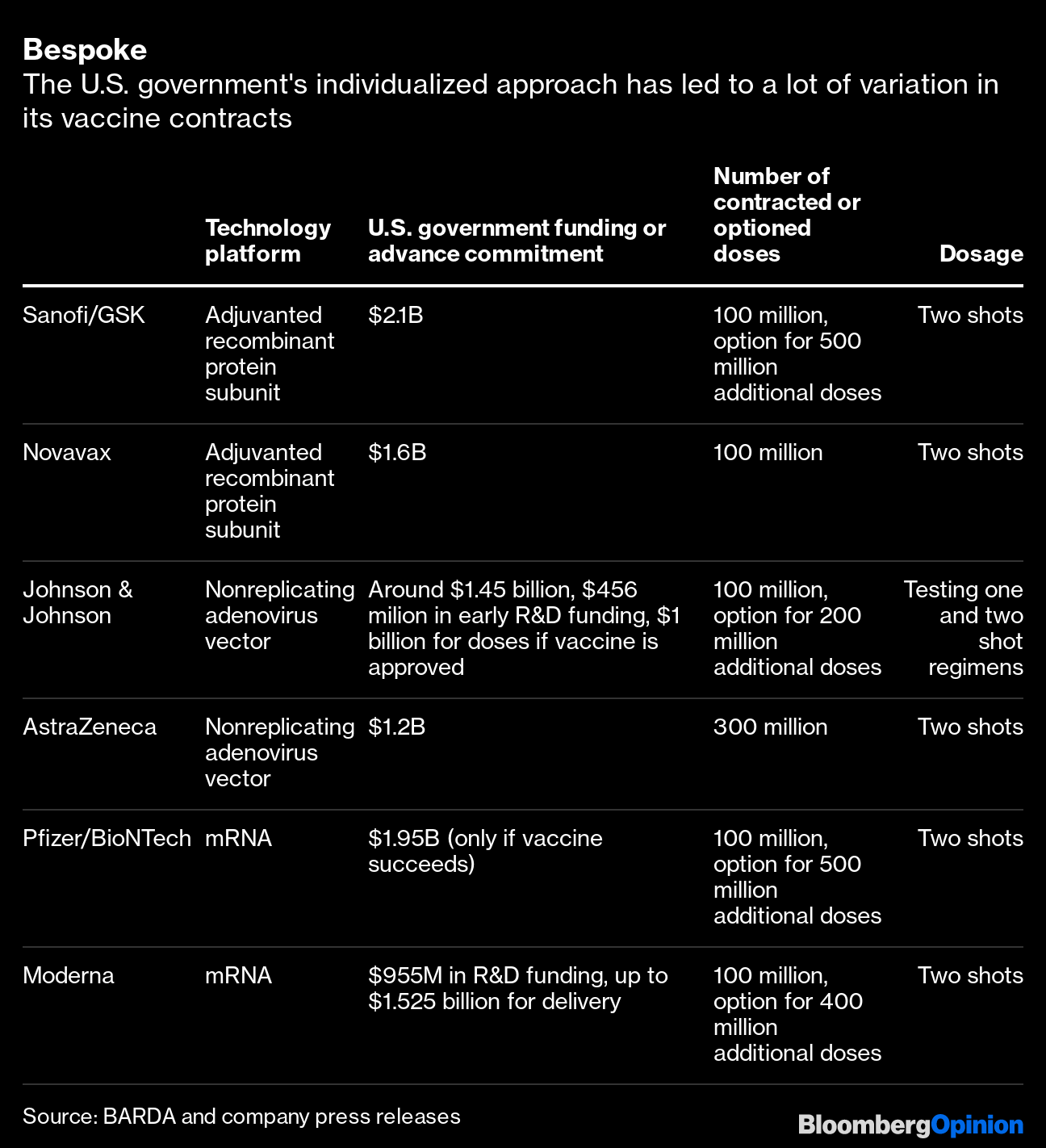
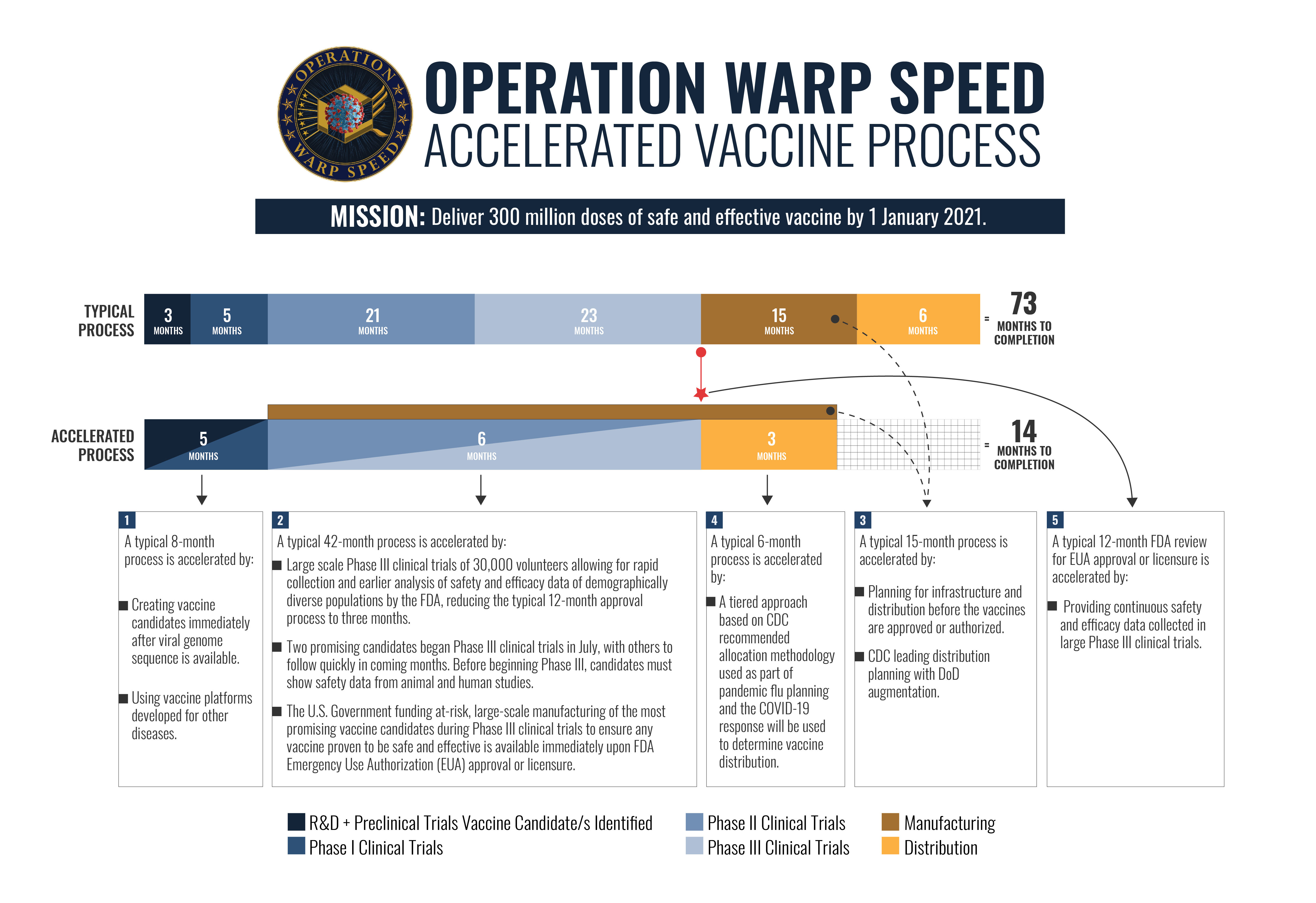
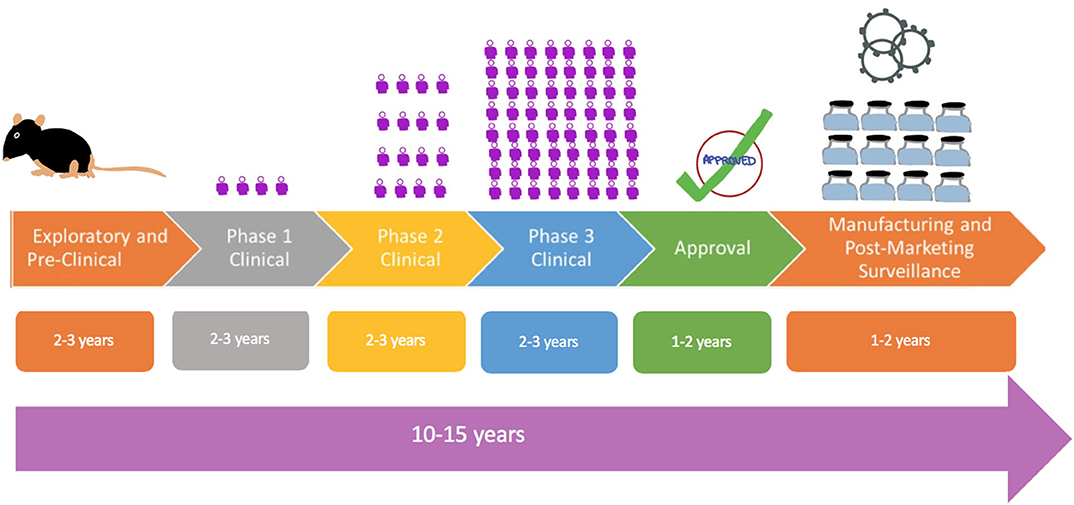
Operation Warp Speed Accelerated Vaccine Process
Pfizer is proud to be one of various vaccine manufacturers participating in operation warp speed as a supplier of a potential covid 19 vaccine the company told the spectator according to adamswhile pfizer did reach an advanced purchase agreement with the us government the company did not accept barda funding for the research and development process.

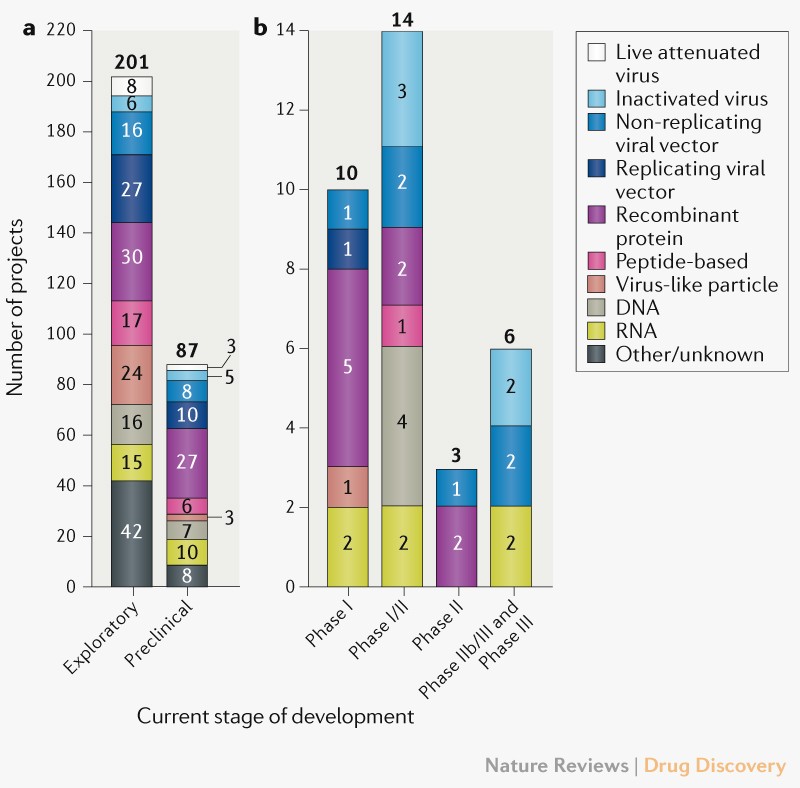
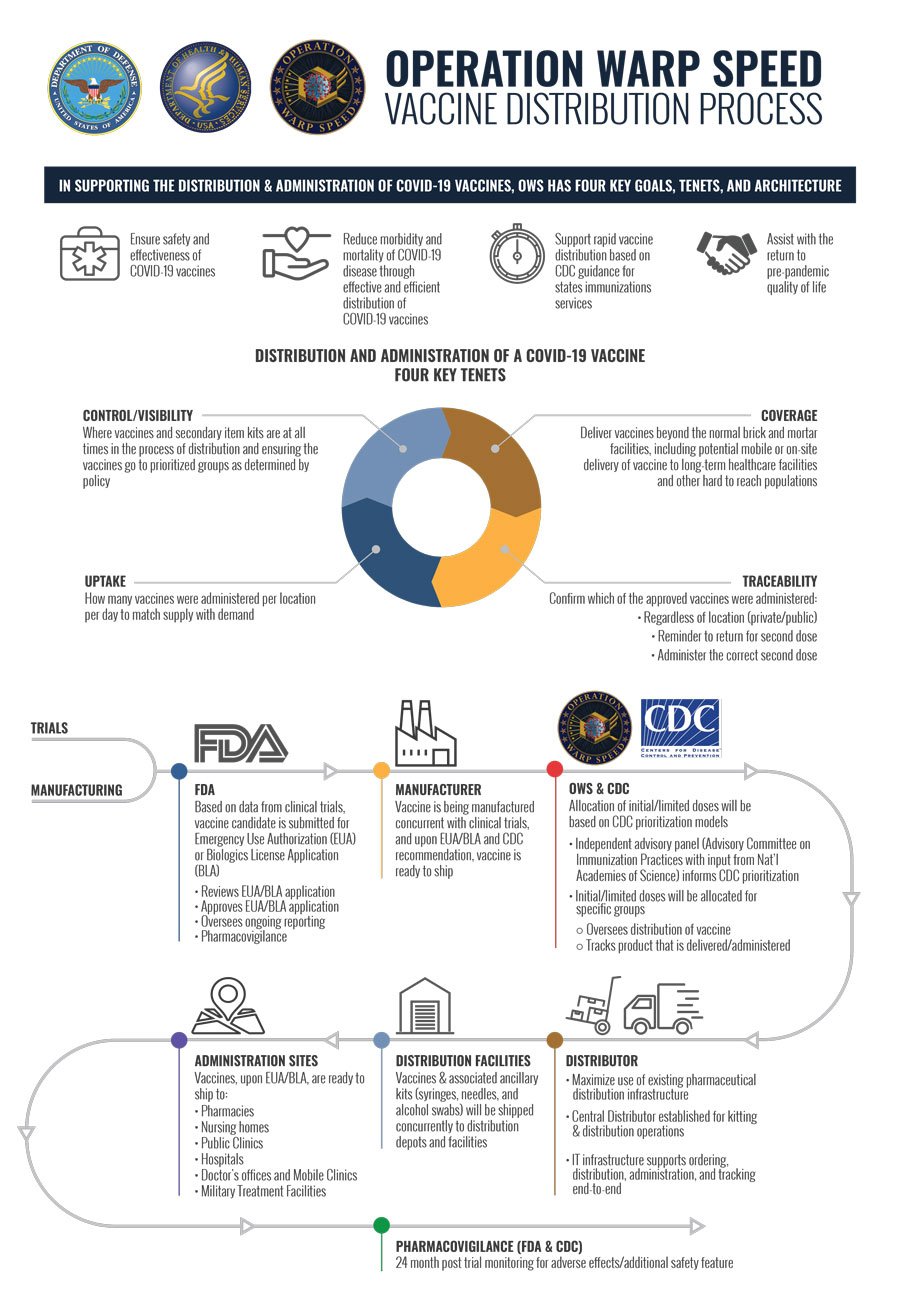
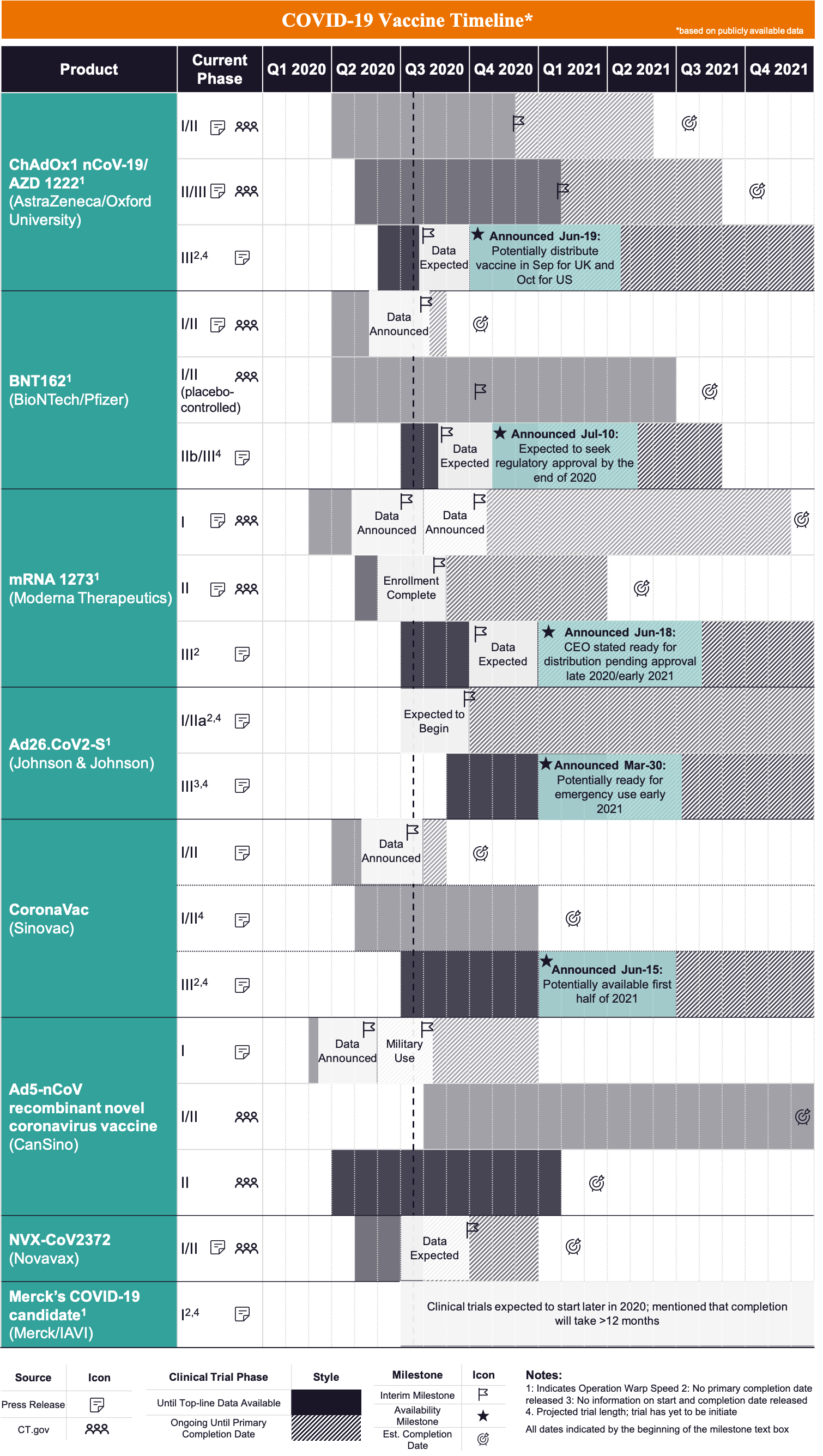
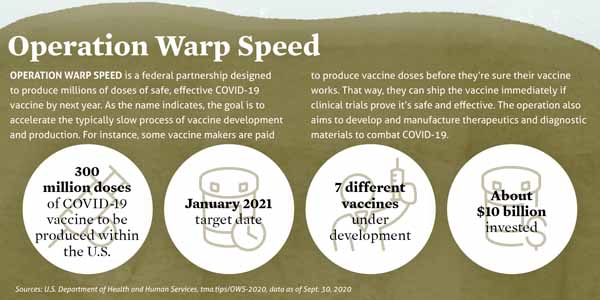
Operation warp speed accelerated vaccine process. Operation warp speed accelerated covid vaccine. Operation warp speeds goal is to produce and deliver 300 million doses of safe and effective vaccines with the initial doses available by january 2021 as part of a broader strategy to accelerate the development manufacturing and distribution of covid 19 vaccines therapeutics and diagnostics collectively known as countermeasures. In order to improve our. Operation warp speed accelerated covid vaccine.
Private sector operation warp speed ows will accelerate the testing supply development and distribution of safe and effective. Ohio valley residents react to election process. Our goal is to create a safe and engaging place for users to connect over interests and passions. Vaccines from moderna and other operation warp speed candidate vaccines would likely be moved by the medical supply company mckesson which has a contract with the government to distribute covid.
The system was created specifically for operation warp speed and links databases that track every covid 19 vaccine dose from manufacture to patient inoculation. Emergency use authorization eua is an accelerated review and authorization process by the fda that would allow vaccine makers to distribute vaccines that are safe and effective but not fully. Former fda commissioner joins the daily briefing to discuss coronavirus vaccine progress. Using the resources of the federal government and the us.
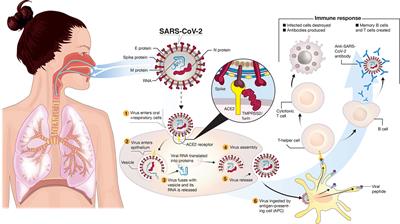
Government to facilitate and accelerate the development manufacturing and distribution of covid 19 vaccines therapeutics and diagnosticsoperation warp speed was introduced in early april 2020 after a round table meeting with industry executives at the white house on march 2.

Trump Picks Ex Drug Company Executive To Lead Accelerated Coronavirus Vaccine Effort The New York Times

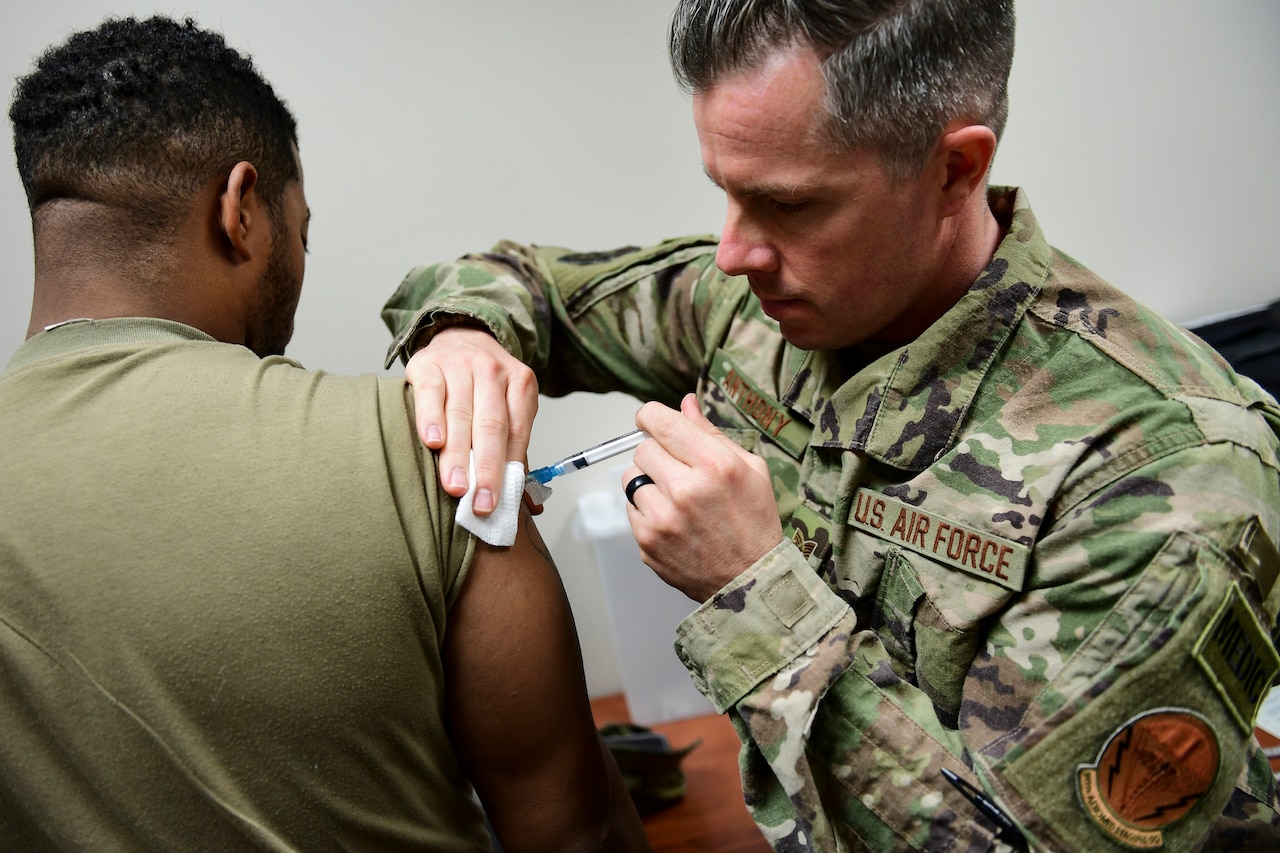





:no_upscale()/cdn.vox-cdn.com/uploads/chorus_asset/file/21943364/AP_20262696421511.jpg)

































:no_upscale()/cdn.vox-cdn.com/uploads/chorus_image/image/67596019/GettyImages_1213154990.0.jpg)


/cdn.vox-cdn.com/uploads/chorus_asset/file/21942877/Screen_Shot_2020_10_07_at_9.28.29_AM.png)